Finding the Ebola virus’ vulnerable points
We know what antibodies stop it in its tracks—we know know where they attach.
by Shalini Saxena Dec 1 2014, 6:00am MPST
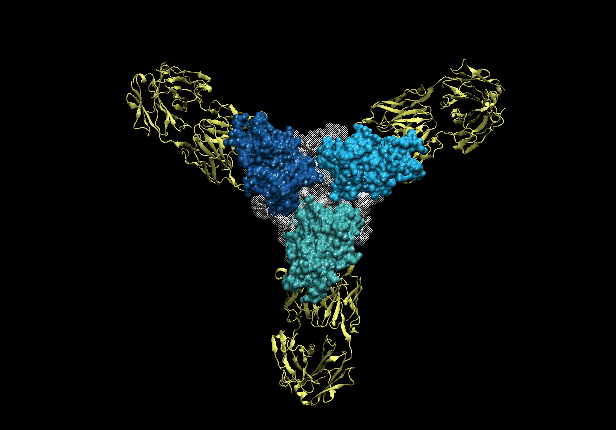
Three copies of the Ebola glycoprotein (blue) with antibodies (yellow) latched on to them.
The latest Ebola outbreak has dwarfed any that have occurred since the discovery of the virus in 1976; previous outbreaks have had lethality rates of up to 90 percent. Yet no vaccines or therapies are currently approved for human use, which limits our ability to treat patients and contain the outbreak. Mixtures of monoclonal antibodies (see sidebar) are a potential treatment option that has been used experimentally.
Monoclonal antibodies bind to a single structural feature on an infectious agent, such as the Ebola virus. These antibodies act as markers to flag down immune cells that destroy the foreign matter. Some antibodies can also be neutralizing, in that they block the harmful biological effects of a virus or prevent the budding of new virus particles. Mixtures of antibodies increase the treatment’s efficacy by limiting the opportunity of a mutant virus to escape recognition.
Two specific monoclonal antibody mixtures have been extensively evaluated: MB-003 and ZMAb. ZMab contains both neutralizing and non-neutralizing antibodies. In contrast, MB-003 antibodies are not neutralizing when administered alone. Recently, both MB-003 and ZMab have been combined into a single treatment named ZMapp that has shown increased efficacy. ZMapp has been successfully administered to human patients who have contracted Ebola during the current outbreak.
Until recently, little has been known about what structural features on Ebola that the antibodies bind to or how that binding occurs. The genetic material of the Ebola virus is protected by a filamentous coating of glycoproteins (GP), which are proteins chemically linked to complex sugars. GP mediates recognition of a host cell, enabling viral entry and subsequent infection. Recently, researchers have investigated how each monoclonal antibody (neutralizing and nonneutralizing) within ZMapp latches on to Ebola. These studies reveal sites of vulnerability on the virus.
Binding competition assays, where antibodies are tested to see if they interfere with each other’s attachment to GP, indicate that the antibodies in ZMapp bind to three general areas on the GP coating. Two antibodies compete strongly at the first area and another two at the second areas, indicating that certain antibodies have overlapping binding sites on the virus surface.
In contrast, two antibodies bind the third domain, yet they do not compete with one another. This suggests that the structural features they bind are physically separate, or that the features are flexible enough to accommodate both antibodies. Researchers believe this area is a mucin-like domain, which has a consistency similar to jelly (the term mucin should evoke mucus).
Single-particle electron microscopy was used to reconstruct the conformations of antibodies bound to a portion of GP to further understand antibody binding in the first two areas. These studies reveal that the first area is at the side/base of GP relative to where it sticks into the virus’ membrane. The second area is the glycan cap, a sugar-coated region that sticks out on the surface like a cap. In both cases, the binding site of these antibodies overlaps extensively and the key difference found was the angle between the antibody and GP.
Overall, these studies reveal how four separate neutralizing antibodies recognize the GP protein. Thus, targeting these three areas of vulnerability on the viral surface in the development of future therapies could lead to enhanced treatments for this deadly disease.
10.1073/pnas.1414164111
Source: http://arstechnica.com/science/2014/11/finding-the-ebola-virus-vulnerable-points/


No comments:
Post a Comment